Cabozantinib is an oral drug which works by blocking the growth of new blood vessels that feed a tumour. In addition to blocking the formation of new blood cells in tumours, Cabozantinib also blocks pathways that may be responsible for allowing cancers cells to become resistant to other “anti-angiogenic” drugs. It is a type of drug called a growth blocker. Cabozantinib has been studied or is already in research studies as a possible treatment for various types of cancer, including prostate cancer, ovarian cancer, brain cancer, thyroid cancer, lung cancer, and kidney cancer. During my research, I found that it has a connection to Medullary Thyroid Cancer (MTC) which is a type of Neuroendocrine Cancer, frequently associated with Multiple Endocrine Neoplasia (MEN). Cabozantinib, under the brand name of ‘Cometriq’ was approved by the FDA in 2012 for use in MTC. Read more about Cometriq here. It’s also been approved by the FDA for advanced renal cell carcinoma (RCC) (branded as Cabometyx). I also discovered that there is an exclusive licensing Agreement with the manufacturers (Elelixis) and Ipsen (of Lanreotide fame) to commercialize and develop Cabozantinib in regions outside the United States, Canada and Japan
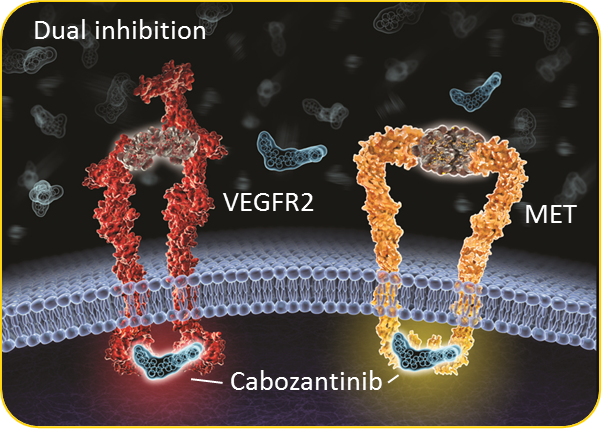
Example action of Cabozantinib
So Capozantinib is a tyrosine kinase inhibitor and is therefore a biological therapy and growth blocker just like Everolimus (Afinitor) and Sunitinib (Sutent). Very technical process but in the simplest of terms, Cabozantinib is deigned to disrupt the actions of VEGF (a growth factor) and MET (a growth factor receptor) which promote spread of cancerous cells through the growth of new blood vessels. Whilst we are on this subject, please note Everolimus (Afinitor) is an mTOR inhibitor and Sunitinib (Sutent) is a tyrosine kinase inhibitor. Many people think these drugs are a type of chemo – that is incorrect, these are targeted biological therapies. See more on this by clicking here.
It’s currently in an ‘Open-Label’ ongoing Phase II Study but is not currently recruiting participants. According to the documentation below, a Phase III trial is in development. The results of the Phase II trial have been presented to both European and US conferences. The trial is confined to Massachusetts in the US in two Boston hospitals (Massachusetts General and Dana-Farber). Dr Jennifer Chan is involved with input from Dr Matthew Kulke – both known NET specialists. The primary study competed in Dec 2016 with a projected study end date of December 2018. According to the trial documentation and informed comment on the trial data to date, the drug targets both Pancreatic Neuroendocrine Tumours (pNETs) and Carcinoid types (e.g. small intestine, appendix, lung, rectum and others). However, these tumours are described as “locally unresectable or metastatic, histologically-confirmed, carcinoid or pancreatic neuroendocrine tumor. Tumors must be considered well- or moderately differentiated (Grade 1 or 2). Patients with poorly differentiated neuroendocrine carcinoma or cell carcinoma are excluded from the study”.
You can read the trial documentation by clicking here.
You can read the output from the poster submission for 2017 Gastrointestinal Cancer Symposium and the output from Onc Live.
Summary
I generated this blog to add value rather than just post the outputs for your own perusal. I hope you find it useful.
Thanks for reading
Ronny Allan
I’m also active on Facebook. Like my page for even more news.
Disclaimer
My Diagnosis and Treatment History
Most Popular Posts
Whatever cancer throws your way, we’re right there with you.
We’re here to provide physical, financial and emotional support.
© Macmillan Cancer Support 2024 © Macmillan Cancer Support, registered charity in England and Wales (261017), Scotland (SC039907) and the Isle of Man (604). Also operating in Northern Ireland. A company limited by guarantee, registered in England and Wales company number 2400969. Isle of Man company number 4694F. Registered office: 89 Albert Embankment, London SE1 7UQ. VAT no: 668265007